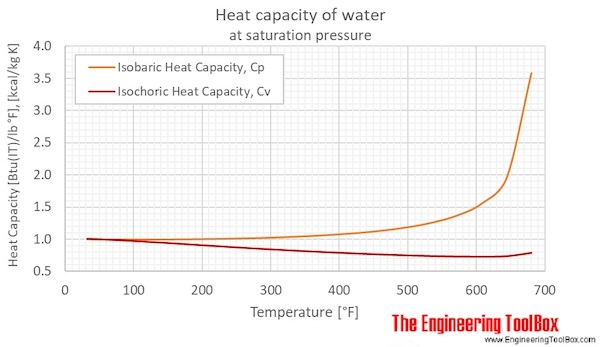
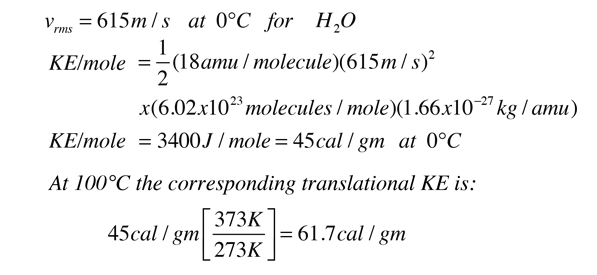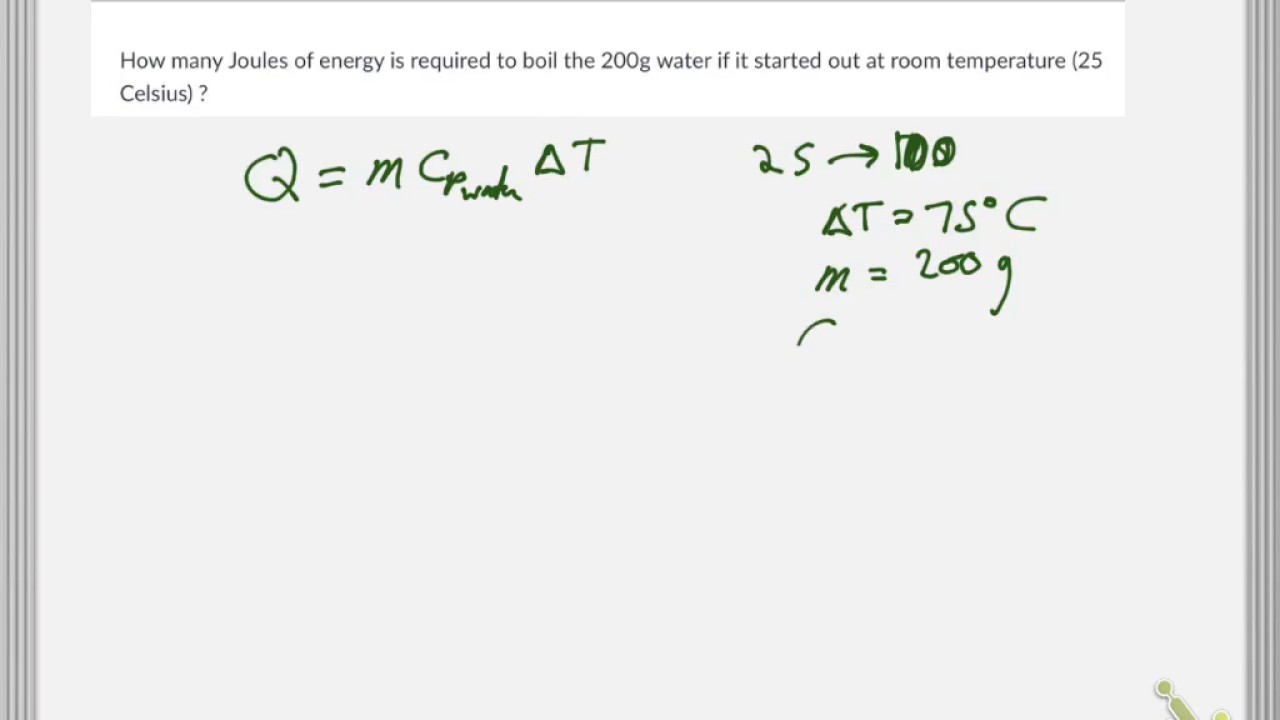Calorie (energy) Calculations A calorie is defined as the amount of energy it takes to raise the temperature of one gram of water by one degree Celsius. - ppt download

The heat energy required to raise the temperature of 2kg of water from 10^0C to 50^0C is (Specific heat capacity of water is 4200 J Kg^0C^-1)

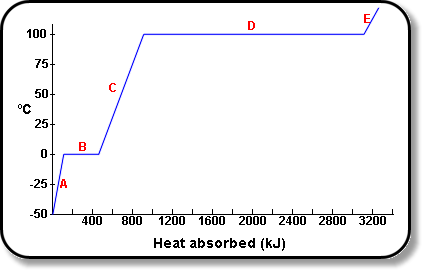
How Much Thermal Energy Is Required To Heat Ice Into Steam - Heating Curve Chemistry Problems - YouTube

SOLVED: Calculate the energy needed to heat 250.0 g of water from 25 degrees * C to 175 degrees * C . Specific heats : water=4.18 J/g^ C,steam=1.99 J/g^ C . A )
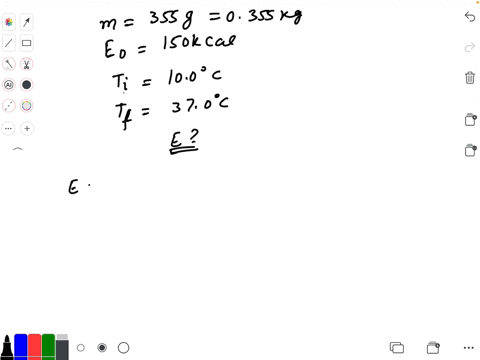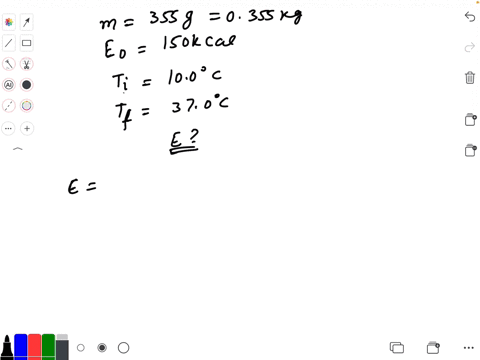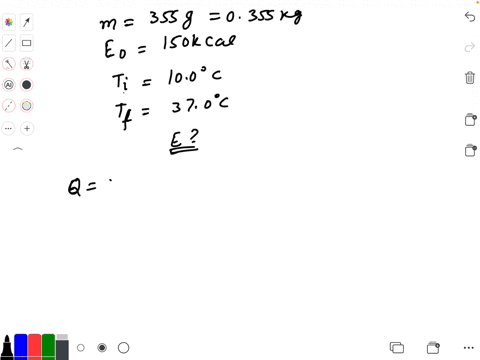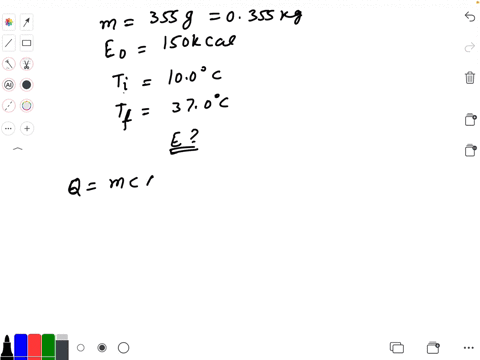
SOLVED: Calculate The Energy Needed To Heat The Cube Of Silver; With A Volume Of Cm? From 14 C To 26 Refer To The Tables Express The Heat In Calories | C&c

Enthalpy. Specific Heat Capacity Definition: The HEAT ENERGY required to raise the TEMPERATURE of 1kg of substance by 1 o C. e.g. for water C= 4.18kJ. - ppt download
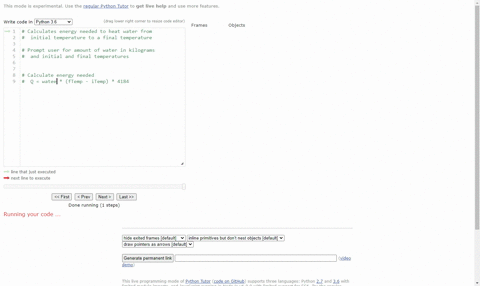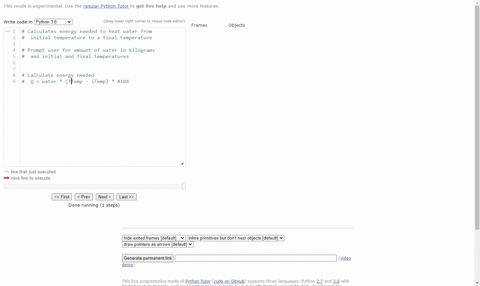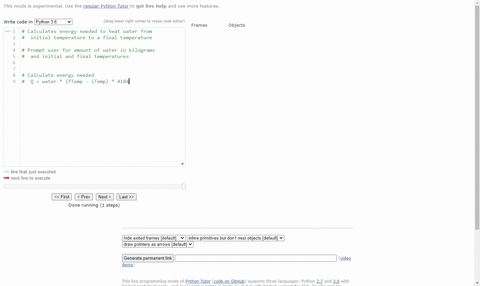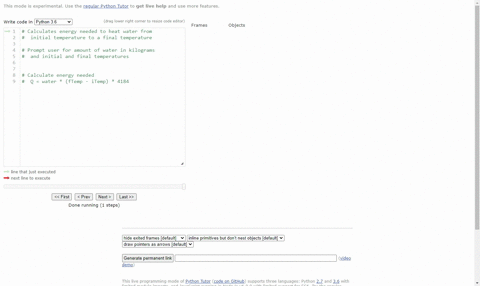
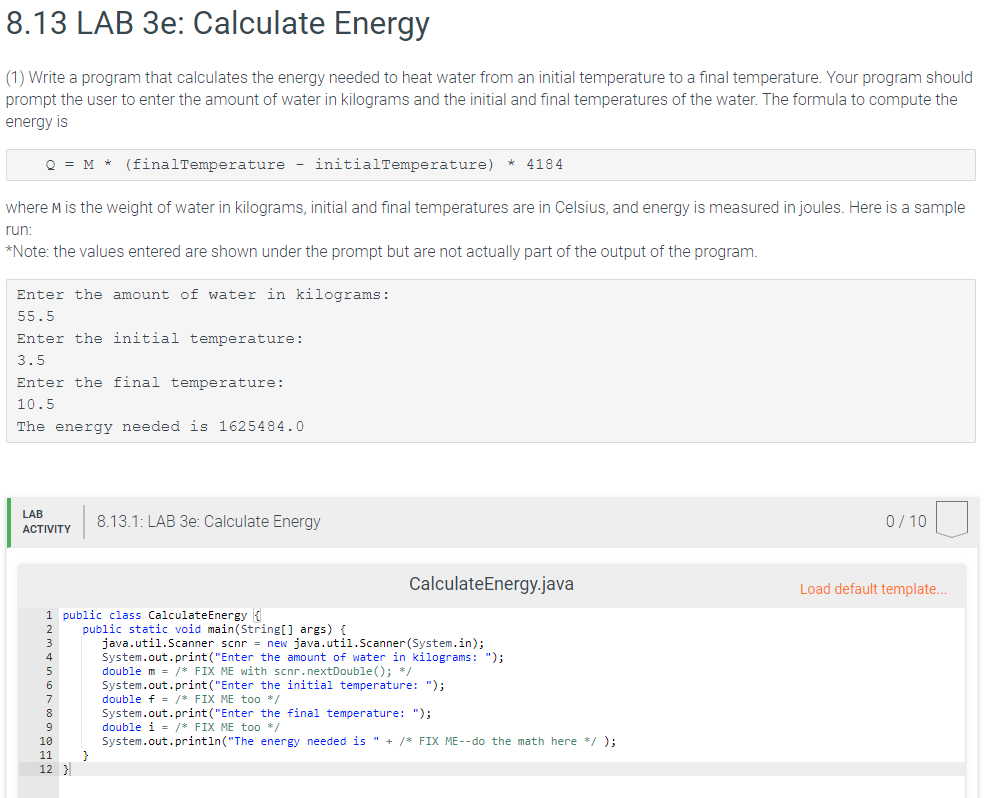
SOLVED:(Science: calculate energy) Write a program that calculates the energy needed to heat water from an initial temperature to a final temperature. Your program should prompt the user to enter the amount